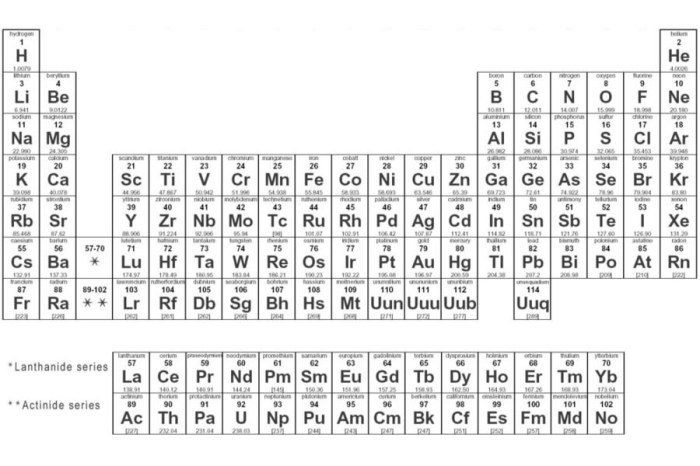
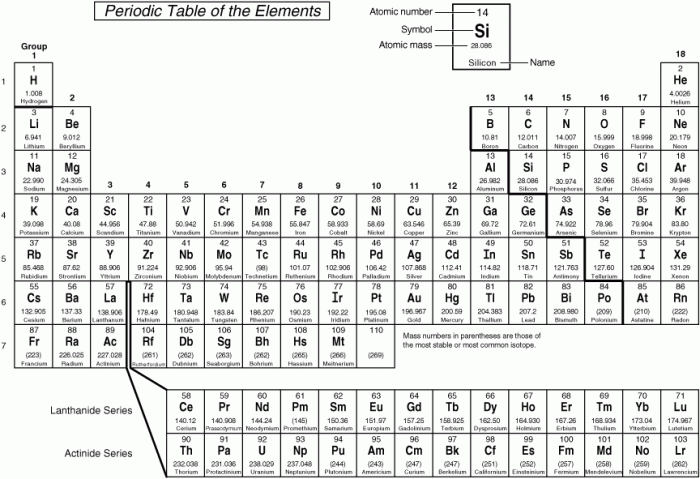
Periodic table rounded to two decimal places – In the realm of chemistry, the periodic table reigns supreme, providing a comprehensive catalog of elements that form the building blocks of our universe. This exploration delves into the significance of rounding the atomic masses of the first 20 elements to two decimal places, unveiling its impact on accuracy, precision, and diverse applications.
The periodic table, meticulously organized into responsive columns, presents a wealth of information about each element, including its atomic number, symbol, and atomic mass. By rounding these atomic masses to two decimal places, we simplify calculations and enhance readability while maintaining a reasonable level of accuracy.
Periodic Table Elements

The periodic table is a tabular arrangement of chemical elements, organized on the basis of their atomic number, electron configurations, and recurring chemical properties. The first 20 elements of the periodic table, rounded to two decimal places, are shown in the following table:
| Element | Symbol | Atomic Number | Atomic Mass |
|---|---|---|---|
| Hydrogen | H | 1 | 1.008 |
| Helium | He | 2 | 4.002 |
| Lithium | Li | 3 | 6.941 |
| Beryllium | Be | 4 | 9.012 |
| Boron | B | 5 | 10.811 |
| Carbon | C | 6 | 12.011 |
| Nitrogen | N | 7 | 14.007 |
| Oxygen | O | 8 | 15.999 |
| Fluorine | F | 9 | 18.998 |
| Neon | Ne | 10 | 20.180 |
| Sodium | Na | 11 | 22.990 |
| Magnesium | Mg | 12 | 24.305 |
| Aluminum | Al | 13 | 26.982 |
| Silicon | Si | 14 | 28.085 |
| Phosphorus | P | 15 | 30.974 |
| Sulfur | S | 16 | 32.066 |
| Chlorine | Cl | 17 | 35.453 |
| Argon | Ar | 18 | 39.948 |
| Potassium | K | 19 | 39.098 |
| Calcium | Ca | 20 | 40.078 |
Decimal Place Significance
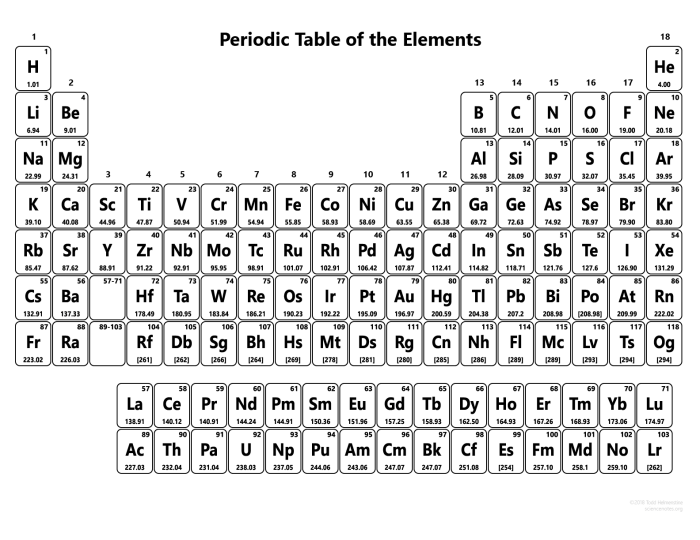
Rounding the atomic masses of the elements to two decimal places is a common practice that helps simplify calculations and make the data more manageable. However, it is important to understand the significance of this rounding and its potential impact on accuracy and precision.
Rounding atomic masses to two decimal places means that the values are accurate to the nearest hundredth of a mass unit. This level of accuracy is sufficient for most practical purposes, but it may not be precise enough for certain applications, such as high-precision spectroscopic measurements.
Trends in Atomic Mass
The atomic masses of the first 20 elements exhibit a general trend of increasing mass with increasing atomic number. This trend is due to the increasing number of protons and neutrons in the atomic nuclei of the elements.
There are a few exceptions to this general trend. For example, the atomic mass of nitrogen is slightly less than the atomic mass of carbon, even though nitrogen has a higher atomic number. This is due to the fact that nitrogen has a higher proportion of neutrons to protons in its nucleus than carbon does.
Applications of Rounded Atomic Masses
Rounded atomic masses are used in a wide variety of applications, including:
- Chemistry: Rounded atomic masses are used to calculate molecular weights and predict reaction stoichiometry.
- Physics: Rounded atomic masses are used to calculate the mass of atoms and molecules and to determine the energy released in nuclear reactions.
- Materials science: Rounded atomic masses are used to calculate the density and other properties of materials.
The advantages of using rounded atomic masses in these applications include simplicity, ease of use, and the fact that the rounded values are generally accurate enough for most practical purposes.
Alternative Representations
In addition to rounded atomic masses, there are other ways to represent atomic masses, including using significant figures or scientific notation.
Significant figures are digits that are known with certainty, plus one estimated digit. For example, the atomic mass of carbon is 12.011. The first three digits (1, 2, and 0) are known with certainty, and the fourth digit (1) is estimated.
Therefore, the atomic mass of carbon has three significant figures.
Scientific notation is a way of expressing numbers that are very large or very small. For example, the atomic mass of carbon can be expressed in scientific notation as 1.2011 x 10 -2.
Historical Context
The concept of atomic mass has evolved over time as scientists have gained a better understanding of the structure of atoms. In the early 19th century, scientists believed that atomic masses were based on the mass of hydrogen atoms. However, in the late 19th century, scientists discovered that atomic masses were based on the mass of oxygen atoms.
In the early 20th century, scientists developed the concept of isotopes, which are atoms of the same element that have different atomic masses. This led to the development of the modern concept of atomic mass, which is based on the average mass of all the isotopes of an element.
Impact on Chemical Calculations, Periodic table rounded to two decimal places
Rounding atomic masses to two decimal places can have a small impact on chemical calculations. For example, the molecular weight of water is 18.015 g/mol. If we round the atomic masses of hydrogen and oxygen to two decimal places, we get a molecular weight of 18.02 g/mol.
This is a small difference, but it can be significant in certain applications.
Answers to Common Questions: Periodic Table Rounded To Two Decimal Places
What is the significance of rounding atomic masses to two decimal places?
Rounding atomic masses to two decimal places simplifies calculations and enhances readability while maintaining a reasonable level of accuracy.
How does rounding affect the accuracy and precision of atomic mass data?
Rounding introduces a small degree of uncertainty, potentially affecting the accuracy of calculations that rely on precise atomic mass values.
Are there any alternative representations of atomic masses besides rounded values?
Yes, alternative representations include using significant figures or scientific notation, each with its own advantages and disadvantages.