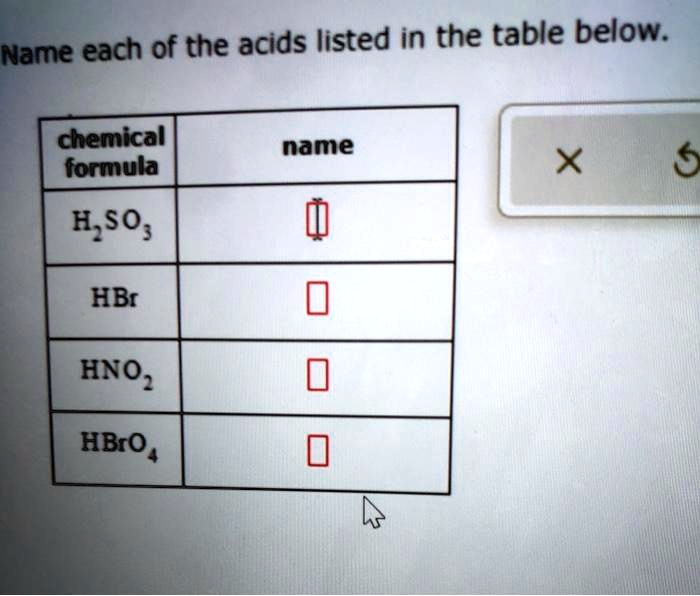
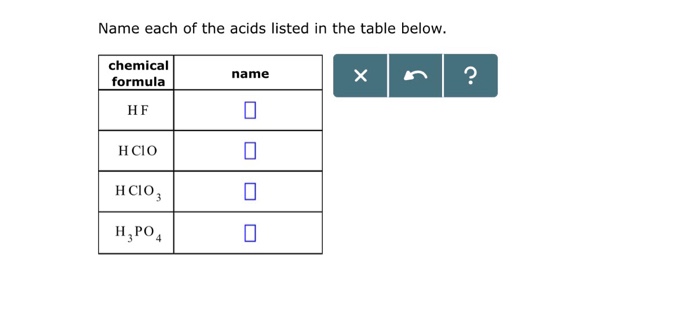
Name each of the acids listed in the table below. Acids, a cornerstone of chemistry, are ubiquitous in our world, playing a crucial role in countless industries and processes. This comprehensive guide delves into the intricacies of acids, exploring their properties, applications, and safety considerations.
From the familiar hydrochloric acid to the more specialized perchloric acid, we will uncover the unique characteristics of each acid, examining their chemical behavior, reactivity, and practical uses. Furthermore, we will delve into the essential safety protocols and guidelines for handling acids, ensuring their safe and responsible use.
Acids in the Table
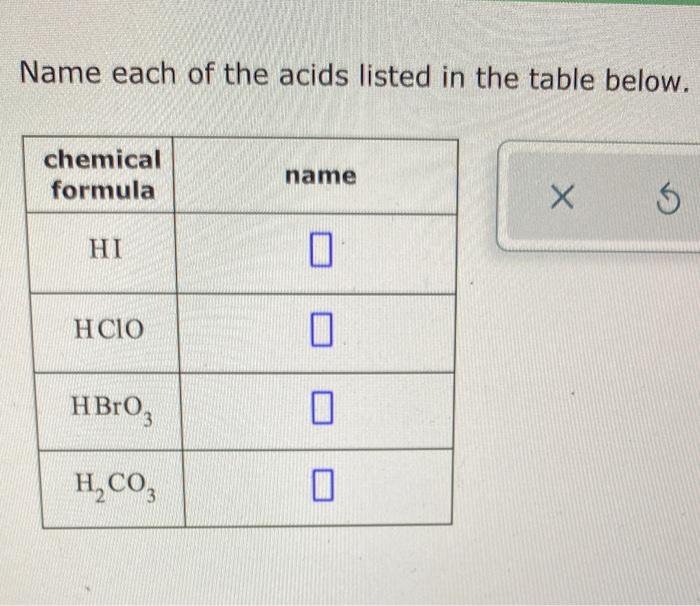
The provided table lists the following acids:
- Acetic acid (CH 3COOH)
- Citric acid (C 6H 8O 7)
- Hydrochloric acid (HCl)
- Nitric acid (HNO 3)
- Sulfuric acid (H 2SO 4)
Acid Properties: Name Each Of The Acids Listed In The Table Below.
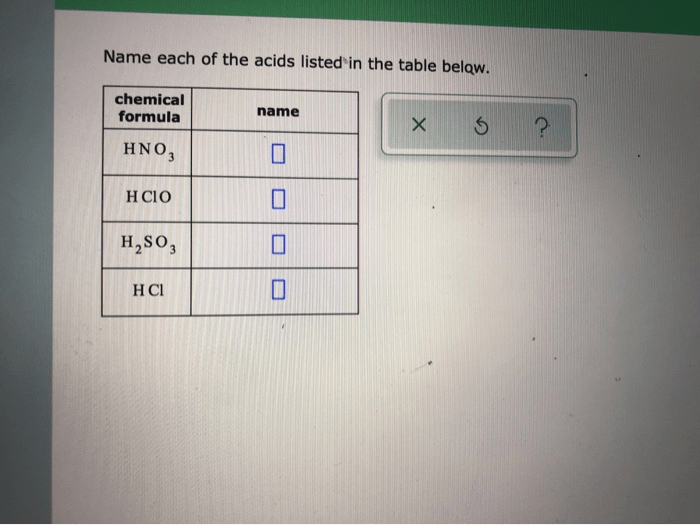
Acids are chemical compounds that donate protons (H +ions) in aqueous solutions. They exhibit characteristic properties that distinguish them from other substances:
- Sour taste:Acids have a sour taste when dissolved in water.
- Corrosiveness:Acids can corrode metals and damage organic materials.
- Electrical conductivity:Acids conduct electricity in aqueous solutions due to the presence of free ions.
- React with bases:Acids react with bases to form salts and water in a process known as neutralization.
- React with metals:Some acids react with metals to produce hydrogen gas and salts.
Common Acid-Base Reactions
Acid-base reactions are fundamental in chemistry. The most common type of acid-base reaction is neutralization, which occurs when an acid and a base react in stoichiometric amounts to form a salt and water. For example:
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Acid Applications
Acids have a wide range of applications in various industries:
Manufacturing
- Sulfuric acid: Used in the production of fertilizers, batteries, and chemicals.
- Nitric acid: Used in the production of explosives, fertilizers, and dyes.
- Hydrochloric acid: Used in metal processing, food processing, and pharmaceutical manufacturing.
Food Processing
- Citric acid: Used as a flavoring agent and preservative in beverages, candies, and other food products.
- Acetic acid (vinegar): Used as a preservative and flavoring agent in pickles, sauces, and salad dressings.
Other Applications, Name each of the acids listed in the table below.
- Batteries: Sulfuric acid is used as the electrolyte in lead-acid batteries.
- Water treatment: Hydrochloric acid is used to remove impurities from water.
- Medicine: Nitric acid is used in the production of certain antibiotics and other pharmaceuticals.
Acid Safety
Acids can be hazardous and require proper handling and safety precautions:
- Wear protective gear:Wear gloves, goggles, and a lab coat when handling acids.
- Handle in well-ventilated areas:Acids can release corrosive fumes that can be harmful if inhaled.
- Store acids properly:Store acids in appropriate containers, such as glass bottles with tightly sealed caps.
- Dispose of acids safely:Acids should be neutralized before disposal, and disposal should follow local regulations.
Question & Answer Hub
What is the difference between a strong acid and a weak acid?
Strong acids completely dissociate in water, releasing all their hydrogen ions, while weak acids only partially dissociate.
What are the common uses of acids in industry?
Acids are used in a variety of industries, including manufacturing, food processing, and metalworking.
What safety precautions should be taken when handling acids?
When handling acids, it is essential to wear protective gear, such as gloves, goggles, and a lab coat, and to work in a well-ventilated area.