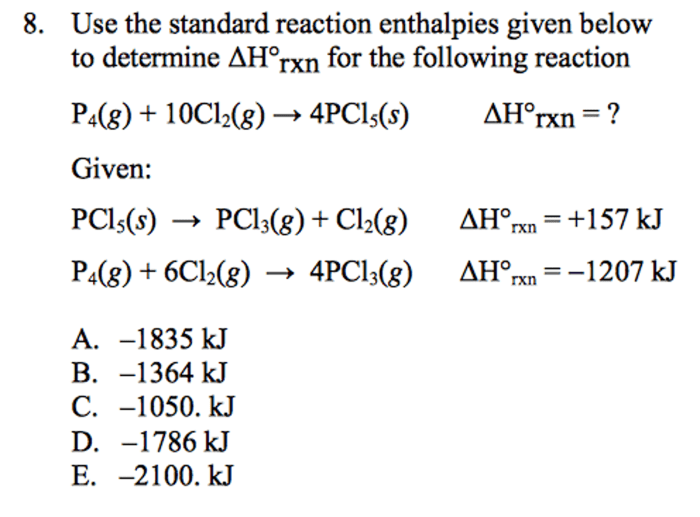
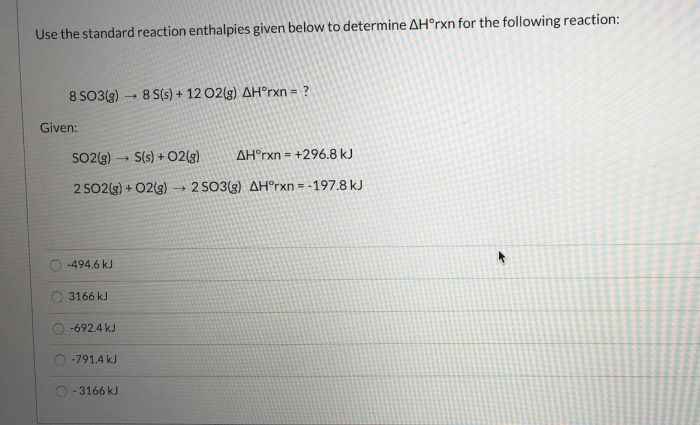
Use the standard reaction enthalpies given below – Harnessing the power of standard reaction enthalpies, this comprehensive guide empowers you to predict reaction spontaneity, calculate equilibrium constants, and delve into the intricacies of thermodynamics and thermochemistry. By delving into the concept, applications, and practical use of standard reaction enthalpies, you will gain a profound understanding of this fundamental aspect of chemistry.
Standard reaction enthalpies serve as a cornerstone in chemistry, providing a quantitative measure of the energy changes associated with chemical reactions. They enable us to comprehend the driving forces behind chemical processes and predict their outcomes with remarkable accuracy.
Standard Reaction Enthalpies: Use The Standard Reaction Enthalpies Given Below
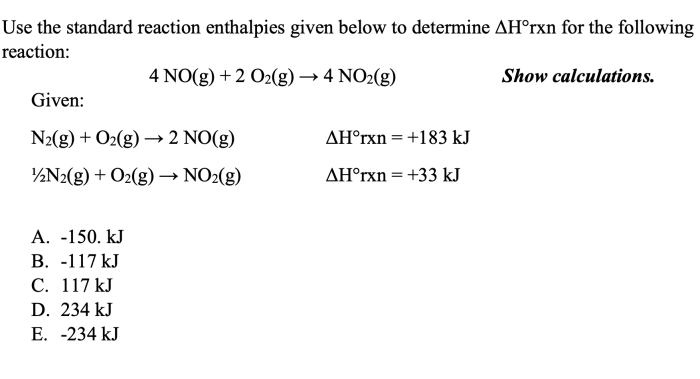
Standard reaction enthalpies, denoted by ΔH°, are important thermodynamic quantities that provide insight into the energetics of chemical reactions. They represent the change in enthalpy, or heat flow, at constant pressure when a reaction is carried out under standard conditions, which are defined as 298 K (25 °C) and 1 atm (101.3 kPa).
Standard reaction enthalpies can be either positive or negative. A positive ΔH° indicates an endothermic reaction, which absorbs heat from the surroundings, while a negative ΔH° indicates an exothermic reaction, which releases heat to the surroundings.
Applications of Standard Reaction Enthalpies
- Predicting the spontaneity of reactions: A negative ΔH° indicates that the reaction is spontaneous, while a positive ΔH° indicates that the reaction is nonspontaneous.
- Calculating the equilibrium constant: The equilibrium constant, K, can be calculated using the ΔH° and temperature.
- Applications in thermodynamics and thermochemistry: Standard reaction enthalpies are used in various thermodynamic calculations, such as determining the heat of formation, heat of combustion, and Gibbs free energy.
Determining Standard Reaction Enthalpies
Standard reaction enthalpies can be determined experimentally using calorimetry. A calorimeter is a device that measures the heat flow associated with a chemical reaction. By measuring the temperature change of the calorimeter and the amount of heat absorbed or released, the ΔH° of the reaction can be calculated.
There are various sources of error and uncertainty in determining standard reaction enthalpies, such as heat losses, incomplete reactions, and instrumental limitations.
Using Standard Reaction Enthalpies in Calculations, Use the standard reaction enthalpies given below
Standard reaction enthalpies can be used to calculate the enthalpy change of a reaction using Hess’s law, which states that the enthalpy change of a reaction is the sum of the enthalpy changes of the individual steps in the reaction.
A table of standard reaction enthalpies can be used to quickly and easily determine the enthalpy change of a reaction.
Extensions and Advancements
Recent advancements in the field of standard reaction enthalpies include the development of new experimental techniques and theoretical models. These advancements have led to a better understanding of the energetics of chemical reactions and their applications in various fields.
Standard reaction enthalpies continue to be an important tool in chemistry, providing valuable insights into the thermodynamics and energetics of chemical reactions.
FAQ Guide
What are standard reaction enthalpies?
Standard reaction enthalpies are the enthalpy changes associated with chemical reactions under specific standard conditions, typically 298 K and 1 atm.
How can I use standard reaction enthalpies to predict reaction spontaneity?
A negative standard reaction enthalpy indicates a spontaneous reaction, while a positive value suggests a non-spontaneous reaction.
What is the relationship between standard reaction enthalpies and equilibrium constants?
The equilibrium constant can be calculated using the standard reaction enthalpy through the Gibbs free energy equation.