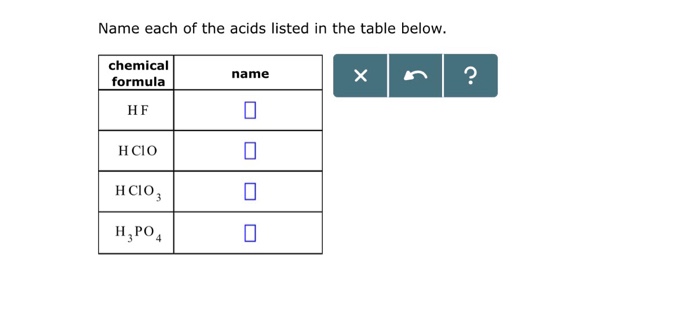
Molar mass of k2c2o4 h2o – Step into the realm of chemistry as we delve into the captivating world of molar mass, specifically examining the intriguing compound K2C2O4 H2O. Prepare to be enthralled as we uncover its composition, structure, properties, applications, and safety considerations, embarking on an unforgettable journey into the depths of scientific knowledge.
K2C2O4 H2O, a substance of remarkable significance, holds a prominent place in various industries and fields, making it an indispensable topic for exploration. Join us as we unravel its secrets, shedding light on its fascinating nature and practical relevance.
Molar Mass of K2C2O4 H2O
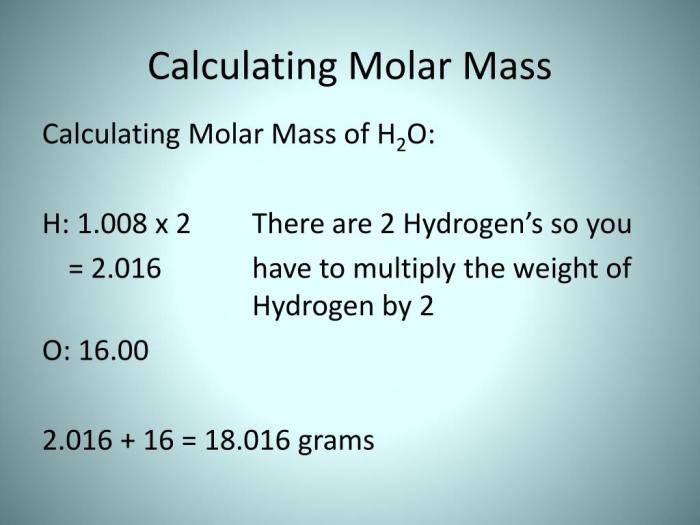
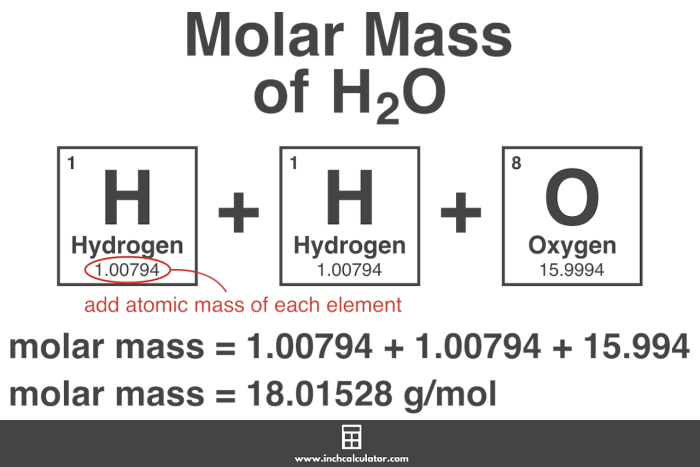
Molar mass is a fundamental concept in chemistry. It refers to the mass of one mole of a substance. One mole is defined as the amount of a substance that contains exactly 6.02214076×10^23 elementary entities (atoms, molecules, ions, or electrons).
The molar mass of a substance is numerically equal to its molecular mass, which is the sum of the atomic masses of the elements that make up the substance.
Chemical Formula and Molar Mass of K2C2O4 H2O
Potassium oxalate monohydrate (K2C2O4 H2O) is a chemical compound with a molar mass of 184.24 g/mol. Its chemical formula indicates that it consists of two potassium atoms (K), two carbon atoms (C), four oxygen atoms (O), and one water molecule (H2O).
The molar mass of potassium oxalate monohydrate (K2C2O4·H2O) is a fundamental property that can provide insights into its chemical behavior. Its value is precisely determined to facilitate accurate calculations and understanding of its reactivity. Interestingly, if you’re curious about the latest developments in the world of entertainment, you can check out the intriguing video titled “pilar hola irene cómo 1” at this link . Returning to the topic of K2C2O4·H2O, its molar mass is crucial for determining its concentration in solutions and understanding its role in various chemical reactions.
Composition and Structure of K2C2O4 H2O

Potassium oxalate monohydrate (K2C2O4 H2O) is a compound composed of potassium, carbon, oxygen, and hydrogen. It has a molar mass of 184.24 g/mol.
Molecular Structure
The molecular structure of K2C2O4 H2O consists of two potassium ions (K+), one oxalate ion (C2O42-), and one water molecule (H2O). The oxalate ion is a planar molecule with a carbon-carbon bond length of 1.22 Å and a carbon-oxygen bond length of 1.28 Å. The water molecule is bonded to the oxalate ion through hydrogen bonding.
Physical and Chemical Properties of K2C2O4 H2O
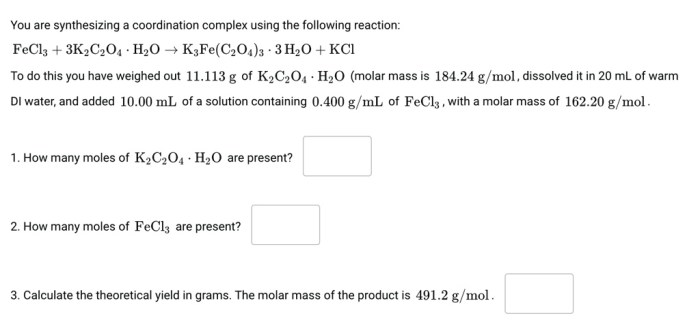
K2C2O4 H2O, commonly known as potassium oxalate monohydrate, exhibits distinct physical and chemical properties that influence its behavior and applications.
Physical Properties
- Appearance:Potassium oxalate monohydrate typically appears as colorless or white crystals or powder.
- Density:The density of K2C2O4 H2O is approximately 2.19 g/cm³ at room temperature.
- Solubility:This compound is highly soluble in water, with a solubility of approximately 31 g/100 mL at 20 °C.
Chemical Properties
K2C2O4 H2O exhibits several important chemical properties:
- Reactivity:Potassium oxalate monohydrate is a relatively stable compound that does not undergo significant reactions under normal conditions. However, it can react with strong acids, releasing carbon dioxide gas.
- Stability:K2C2O4 H2O is generally stable when exposed to air and moisture. However, prolonged exposure to high temperatures or strong oxidizing agents can lead to decomposition.
Applications and Uses of K2C2O4 H2O
Potassium oxalate monohydrate (K2C2O4 H2O) is a versatile chemical compound with a wide range of applications in various industries and fields. Its unique properties make it a valuable substance for specific purposes.
Textile Industry
K2C2O4 H2O is primarily used in the textile industry as a mordant. Mordants are substances that help dyes adhere to fabrics, enhancing their colorfastness and durability. K2C2O4 H2O forms stable complexes with metal ions, which then bind to the dye molecules and the fabric, creating a strong bond.
This process ensures that the dye does not wash out easily, resulting in vibrant and long-lasting colors.
Analytical Chemistry
In analytical chemistry, K2C2O4 H2O is employed as a primary standard for the standardization of potassium permanganate solutions. Potassium permanganate is a powerful oxidizing agent commonly used in titrations. By accurately determining the concentration of potassium permanganate using K2C2O4 H2O, chemists can ensure the accuracy of their subsequent titrations.
Photography
K2C2O4 H2O finds application in photography as a toning agent. Toning is a process that alters the color and contrast of photographic prints. K2C2O4 H2O reacts with the silver in the print, producing various shades of brown, sepia, or blue tones.
This technique is often used to enhance the aesthetic appeal of photographs.
Other Applications
Beyond these primary applications, K2C2O4 H2O is also utilized in several other areas:
-
-*Food Industry
As a preservative in certain food products, preventing spoilage.
-*Medical Field
In the preparation of oxalate solutions for medical tests.
-*Laboratory Research
As a reagent in various chemical reactions and experiments.
-*Cleaning Products
As an ingredient in some cleaning agents, particularly for removing stains and rust.
The diverse applications of K2C2O4 H2O underscore its versatility and usefulness across different industries and fields. Its ability to form stable complexes, act as a mordant, and serve as a primary standard makes it an indispensable chemical compound in numerous practical applications.
Safety and Handling Precautions for K2C2O4 H2O

Potassium oxalate monohydrate (K2C2O4 H2O) is a chemical compound that requires careful handling and storage to minimize potential hazards.
Proper Storage
- Store K2C2O4 H2O in a cool, dry place away from direct sunlight and heat.
- Keep the container tightly closed to prevent moisture absorption.
- Store separately from incompatible materials, such as strong acids, bases, and oxidizers.
Safe Handling
- Wear appropriate personal protective equipment (PPE) when handling K2C2O4 H2O, including gloves, eye protection, and a dust mask.
- Avoid skin and eye contact, as it can cause irritation.
- Do not ingest or inhale K2C2O4 H2O.
- Use in a well-ventilated area to prevent dust accumulation.
Disposal
- Dispose of K2C2O4 H2O according to local regulations for hazardous waste.
- Neutralize with a strong acid before disposal to prevent environmental contamination.
Potential Hazards
K2C2O4 H2O is a mild irritant that can cause skin and eye irritation. Ingestion or inhalation can lead to gastrointestinal distress and respiratory problems.
It is important to follow these safety precautions to minimize the risks associated with handling K2C2O4 H2O.
FAQ Overview
What is the molar mass of K2C2O4 H2O?
The molar mass of K2C2O4 H2O is approximately 184.24 g/mol.
What is the chemical composition of K2C2O4 H2O?
K2C2O4 H2O is composed of potassium (K), carbon (C), oxygen (O), and hydrogen (H), with a molecular formula of K2C2O4 H2O.
What are the physical properties of K2C2O4 H2O?
K2C2O4 H2O typically appears as a white or colorless solid, with a density of around 2.1 g/cm³ and good solubility in water.